By Joanne Moody, Zeta Scientific LLC, Livermore, CA
Wearable medical devices are skyrocketing based on sensor developments and remote monitoring by both consumers and medical professionals. New streamlined devices and drug delivery are small, lightweight, and directly attached to the skin with pressure sensitive adhesives (PSAs). PSAs require just light pressure to stick. Adhesives used are acrylics, silicones, synthetic rubbers, hydrocolloids, and hydrogels - all needing to pass biocompatibility requirements. Examples of devices are below.
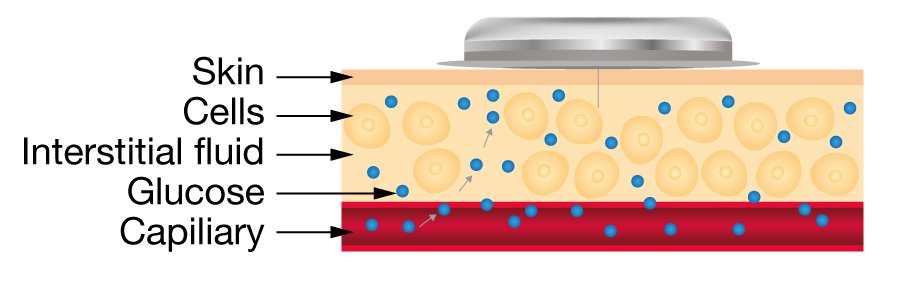
Glucose Monitoring Device
Glucose monitoring devices eliminate the need to prick the skin for blood and glucose measurements.
Based on an image from Image source: https://www.freestylelibre.us/?source=freestylelibre.com
Transdermal Drug Delivery Patch
Transdermal drug delivery patches deliver drugs through the skin, needle-free. It offers the advantage of not having the drug destroyed in the stomach.
Transdermal Skin Patch Construction
A range of patch designs includes multilayer constructions with matrix, reservoir, and drug in the adhesive. The drug might passively enter the skin or have an active delivery enhancement involving ultrasound, electrically assisted methods, jet injectors, thermal approaches, and microneedles.
Multilaminate
Challenges
Topical skin adhesive patches have challenges, including sticking firmly to the human skin, removal without skin irritation, and leaving no residue. A long-term wearable patch is currently 14 days. Design considerations are skin area, age of skin, size/weight of the device, flexibility, wear duration, breathability, environment, application, and removal method. The skin adhesive and device must meet ISO 10993 biocompatibility standards.
Summary
Skin patch applications are proliferating based on sensor developments and remote monitoring by consumers and medical professionals who want instant information. Skin sensitivity and integration of adhesives into the sensor design will be a continuous challenge. These devices help with patient compliance for better outcomes.
About the Author
Joanne Moody is Principal Consultant at Zeta Scientific LLC. She specializes in solving complex, real world challenges involving adhesives, coatings, plastics, and materials science. Clients are startups to international corporations. Industry experience include medical devices, consumer products, electronics, transportation, food, chemical, and water filtration.